How to Draw Line Angle Structures Easily
11.3: Condensed Structural and Line-Angle Formulas
-
- Last updated
- Save as PDF
- Page ID
- 83133
Skills to Develop
- Write condensed structural formulas for alkanes given complete structural formulas.
- Draw line-angle formulas given structural formulas.
We use several kinds of formulas to describe organic compounds. A molecular formula shows only the kinds and numbers of atoms in a molecule. For example, the molecular formula C4H10 tells us there are 4 carbon atoms and 10 hydrogen atoms in a molecule, but it doesn't distinguish between butane and isobutane. A structural formula shows all the carbon and hydrogen atoms and the bonds attaching them. Thus, structural formulas identify the specific isomers by showing the order of attachment of the various atoms.
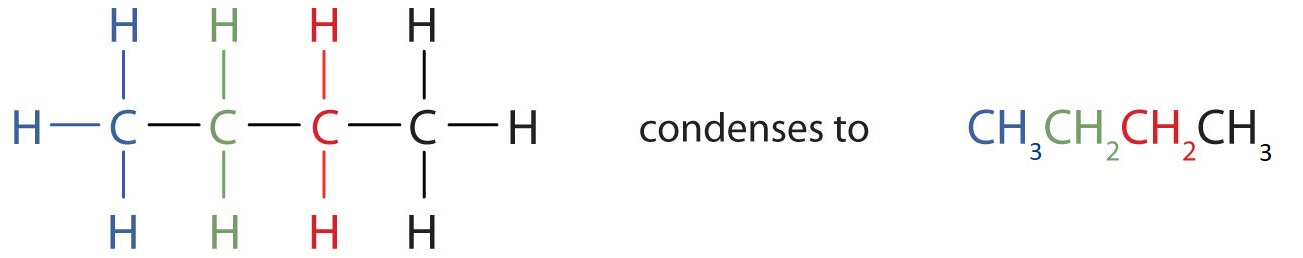
Unfortunately, structural formulas are difficult to type/write and take up a lot of space. Chemists often use condensed structural formulas to alleviate these problems. The condensed formulas show hydrogen atoms right next to the carbon atoms to which they are attached, as illustrated for butane:
Even more abbreviated is a line-angle formula, also called a skeletal structure, in which carbon atoms are implied at the corners and ends of lines, and each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds. All other types of atoms are shown and hydrogens bonded to atoms other than carbon are shown. For example, we can represent pentane (CH3CH2CH2CH2CH3) and isopentane [(CH3)2CHCH2CH3] as follows:

Parentheses in condensed structural formulas indicate that the enclosed grouping of atoms is attached to the adjacent carbon atom.
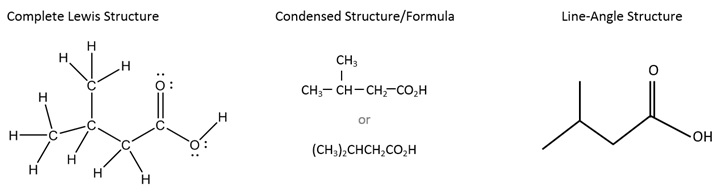
Below is an example of a more complicated molecule. On the left is shown the complete Lewis structure, showing all atoms and valence electrons. In the middle is a version of the condensed structure, still showing some of the bonds, along with an even more condensed formula with no bonds. Finally, on the right is the line-angle (skeletal) structure; notice that the bonds are shown, but not the carbons and not the hydrogens bonded to carbon.
The following are suggested steps for drawing a line-angle structure (also known as a skeletal structure).
- Remove all hydrogens bonded to carbons.
- Remove all carbons.
- Because the carbons on the left are drawn straight across, we cannot see corners easily, so bend your lines (zig-zag) so that the corners are apparent.
Key Takeaways
- Condensed chemical formulas show the hydrogen atoms (or other atoms or groups) right next to the carbon atoms to which they are attached.
- Line-angle formulas imply a carbon atom at the corners and ends of lines. Each carbon atom is understood to be attached to enough hydrogen atoms to give each carbon atom four bonds.
Exercises
1. Write the condensed formula for each Lewis structure.
A.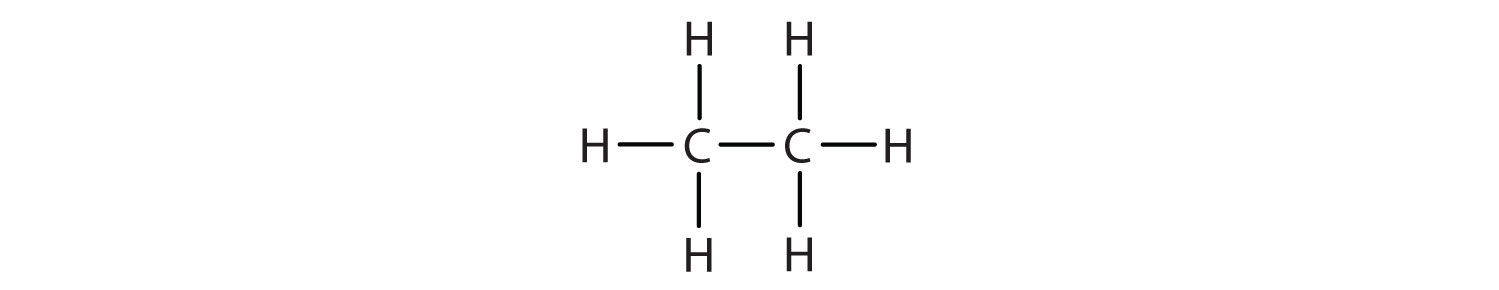
B.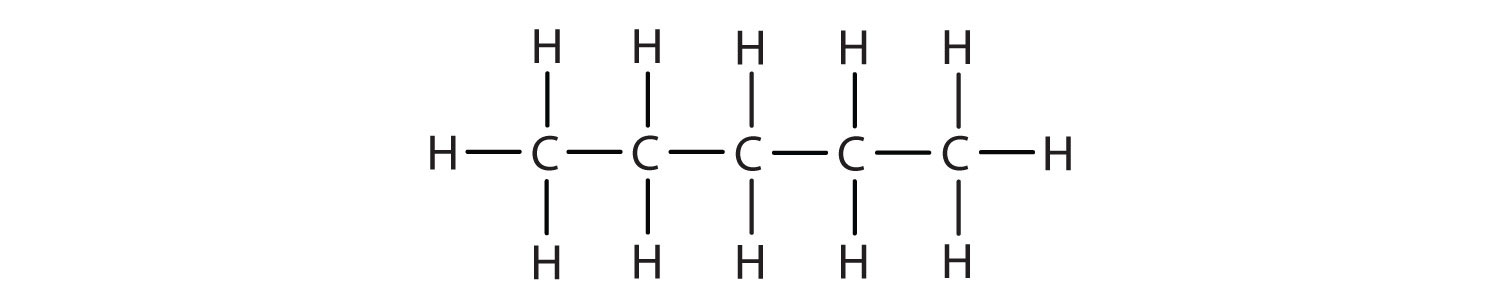
2. Draw a line-angle structure for the compound CH3CH2CH(CH3)CH2CH2CH3.
3. Give the condensed formula for the compound represented by this line-angle structure:
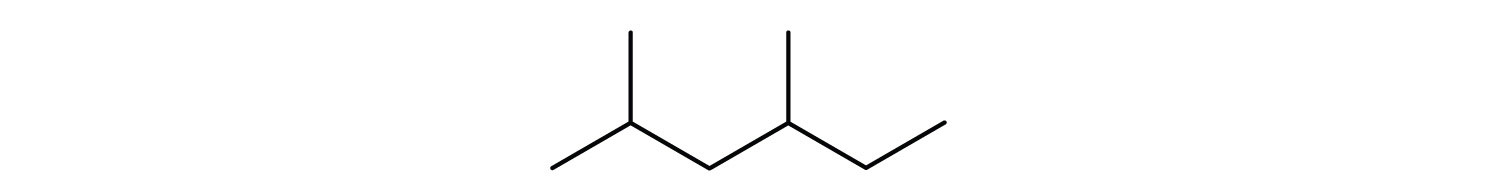
4. Draw the Lewis structure of the molecule below, showing all atoms and all valence electrons (bonds and lone pairs).
.jpg?revision=1)
5. Draw the Line-Angle structure for the molecule below.
.jpg?revision=1)
Answers
- A. CH3CH3 B. CH3CH2CH2CH2CH3
2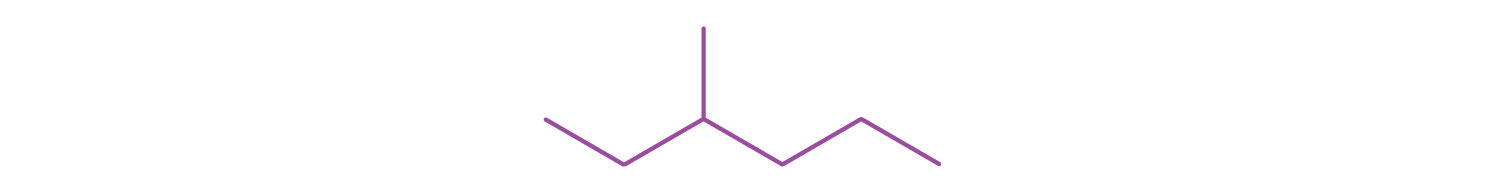
3. (CH3)2CHCH2CH(CH3)CH2CH3
4.
.jpg?revision=2)
5.
.jpg?revision=1)
How to Draw Line Angle Structures Easily
Source: https://chem.libretexts.org/Courses/University_of_South_Carolina__Upstate/USC_Upstate%3A_CHEM_U109_-_Chemistry_of_Living_Things_(Mueller)/11%3A_Organic_Chemistry/11.03_Condensed_Structural_and_Line-Angle_Formulas

0 Response to "How to Draw Line Angle Structures Easily"
Post a Comment